

The pharmaceutical watchdog has been urged to investigate after a string of celebrity doctors took part in discussions about the Covid vaccine but failed to declare they had been paid by AstraZeneca.
In recent weeks, several high profile doctors have taken part in debates about the company’s vaccine on primetime television shows, but viewers were not informed that they have previously received thousands of pounds from the pharmaceutical giant.
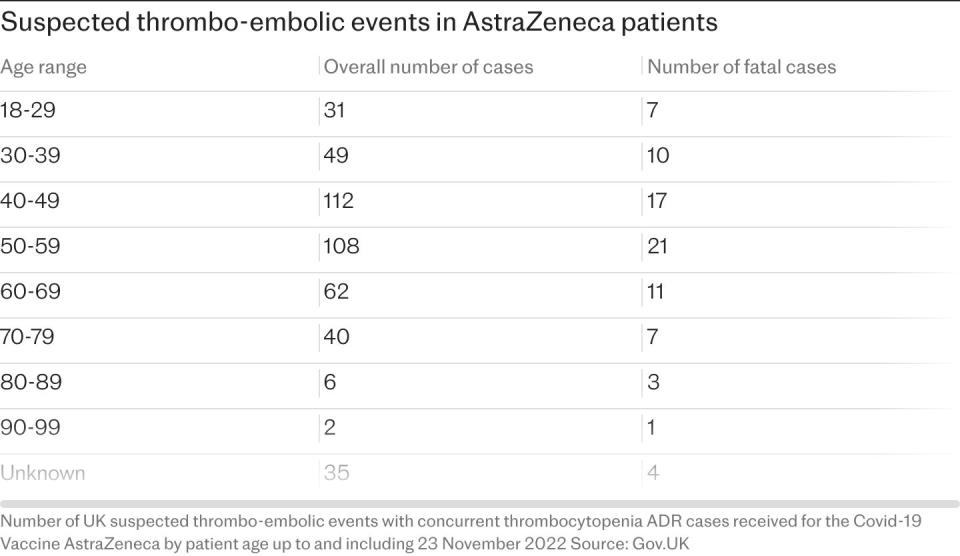
In April of this year, AstraZeneca admitted for the first time in court documents that its Covid vaccine can, in a small percentage of cases, cause a rare and dangerous side effect. The following month, it emerged that the Oxford-AstraZeneca Covid vaccine is being withdrawn worldwide.
The revelations prompted a fresh round of debate about the vaccine, with celebrity doctors invited on to television shows to discuss the fallout.
Dr Ranj Singh, who regularly appears on the BBC as a talking head, was paid £22,500 by AstraZeneca in 2021, according to records from the Association of the British Pharmaceutical Industry (ABPI).
Last month, he led a discussion on the BBC breakfast show Morning Live about the safety of the AstraZeneca vaccine and the “serious but rare” complications associated with it. He failed to declare his payments from the pharmaceutical giant to either the BBC or to viewers.

A BBC spokesman said they were unaware of the payments ahead of the show, adding: “The segment on the AstraZeneca Covid vaccine was balanced and covered reported risks and benefits. We became aware of Dr Ranj’s 2021 work for the manufacturer after this segment aired and have now addressed this within the show.”
Dr Nighat Arif, who became a familiar face on television during the pandemic including on BBC Breakfast, was paid £10,000 by Astra-Zeneca in 2022.
In April, she appeared on ITV’s This Morning to discuss possible side effects of the AstraZeneca vaccine such as blood clots, and reassured people that these are “very, very rare”.
An ITV spokesman said that Dr Arif’s analysis was “fair, accurate and balanced”, adding that: “In her capacity as a medical professional [she] described the scientific process by which the vaccine could cause a blood clot.
“She indicated that this was ‘rare’ on the basis that the number of people who had been identified as having suffered a clot as a result of the AstraZeneca vaccine was statistically small in comparison with the millions of people who had received the vaccine. This is in accordance with information published by the Government this year. She also referenced the Pfizer and Moderna vaccines within the discussion.”
Dr Phillipa Kaye was paid £12,500 by AstraZeneca in 2020 and a further £9,000 by the same company in 2022.

During the pandemic she was prominent on social media, posting videos of herself encouraging people to get the Covid vaccine, some of which were officially promoted by the Department of Health.
In one post on X, formerly Twitter, in April 2021 she told her followers about the “benefit vs risk for the AZ vaccine”, explaining: “Risk of dying from a clot with the vaccine is one in 2.5 million. If 2.5 million 40-year-old gets Covid 2,000 will die and one in 20 (125,000 people) will have long Covid. Benefit outweighs risk.”
Alex Fell, director of the pharmaceutical regulator, has been urged to investigate whether there have been any breaches of the ABPI code of practice for pharmaceutical companies.
The letter, sent from Graham Stringer and Lord Strathcarron, points out that Clause 24 of the ABPI code requires pharmaceutical companies to include provisions relating to disclosure of such payments when they draw up contracts with doctors.
The code says that “in their written contracts or agreements, companies must include provisions regarding the obligation of the individual to declare that they are a contracted individual to the company whenever they write or speak in public about a matter that is the subject of the agreement or any other issue relating to that company”.
Breaches of the code are investigated by the Prescription Medicines Code of Practice Authority (PMCPA).

Mr Stringer and Lord Strathcarron, who are co-chairs of the all-party parliamentary group on pandemic response and recovery, say that action is required by the watchdog “to maintain trust in advice from doctors”.
They say: “Whether via the TV or in the GP surgery, any payments made to them by pharmaceutical companies should be publicly registered and fully disclosed. If a potential conflict of interest is concealed from the public, it is inevitable that trust will be diminished, which is bad for everyone.”
All of the doctors have issued similar statements to explain that they were not paid by AstraZeneca specifically in relation to the Covid vaccine. They say that they were paid – via a public relations company – to promote the pharmaceutical giant’s nasal flu vaccine.
Dr Nighat Arif said: “I worked with Astrazeneca (via a PR agency) on an educational public health campaign about the nasal flu vaccine in winter 2021 only. All payments were declared on the ABPI. To make it absolutely clear I have not been paid to promote the Covid vaccine.”
Dr Ranj Singh said: “The AZ Oxford vaccine was launched in 2020/21. I worked with AZ (via a PR company) on an entirely separate childhood flu campaign in 2022 which was declared to the ABPI. And I have not worked with them since then. I have never been paid to promote Covid vaccines.”
Dr Phillippa Kaye said: “I have worked with Astrazeneca (via PR agencies) on educational campaigns about the nasal flu vaccine only. The last time I was involved in the nasal flu vaccine education campaign was winer 2021. All payments were declared to the ABPI. To be clear, I have not been paid to promote Covid vaccines.”
AstraZeneca declined to comment on the specific cases, but pointed out that this is an area governed by the ABPI’s code of practice which applies to the whole pharmaceutical industry.
Dr Amit Aggarwal, the ABPI’s executive director of medical affairs, said they are “committed to transparency in the relationships between pharmaceutical companies and NHS professionals, and proud that Disclosure UK helps provide that transparency”.
EMEA Tribune is not involved in this news article, it is taken from our partners and or from the News Agencies. Copyright and Credit go to the News Agencies, email news@emeatribune.com Follow our WhatsApp verified Channel









